The role of clinical trials networks in evidence generation in Australia and New Zealand
Clinical trial evidence comes from a variety of sources. In Australia and New Zealand, a large proportion of that evidence comes from Clinical Trial Networks (CTNs). These groups of geographically dispersed and multidisciplinary clinical researchers play an important role in evidence generation, particularly for investigator-initiated trials.
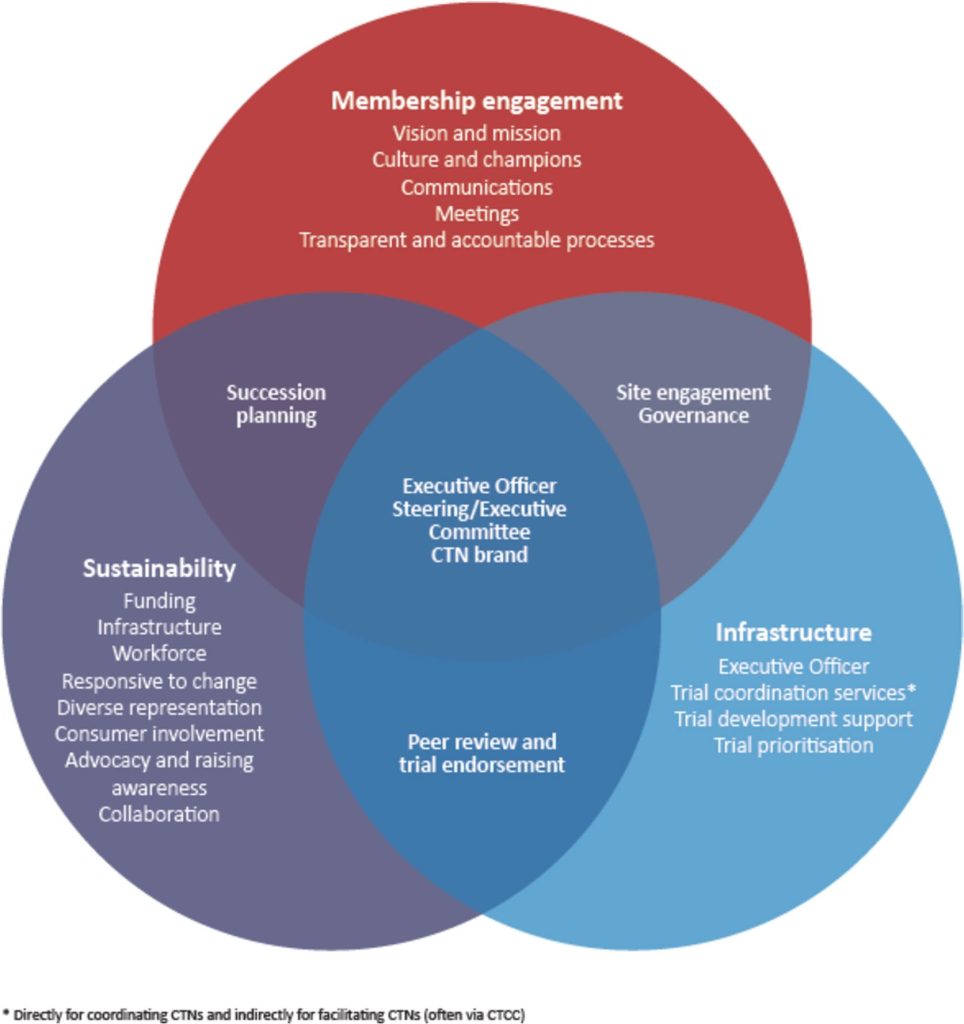
A group of researchers from Australia and New Zealand has just published a paper in Trials that has identified three key themes associated with the success and growth of a Clinical Trial Network. These include:
- Engaged membership
- Established infrastructure
- Sustainability
The paper suggests that by supporting both new and established Clinical Trial Networks, the efficiencies of these organizations can be improved. This is particularly important in a resource-constrained sector.
We congratulate the authors on their publication and look forward to seeing how the implementation of these critical success factors can increase the return on investment in this sector.
WriteSource Medical Pty Ltd was proud to support the team by providing medical writing assistance for this work.
The figure accompanying this blog is Figure 1 from: Activities critical to success and growth of clinical trials networks. What is needed and how are we doing? An Australian and New Zealand perspective and is reproduced under the Creative Commons CC BY license.


Comments are closed.